Insights for Oncology Marketing
February 23, 2023
Are you taking full advantage of your medical partners?
KRISTEN AMBEGAOKAR, PHD | MEDICAL DIRECTOR

February 23, 2023
Are you taking full advantage of your medical partners?
KRISTEN AMBEGAOKAR, PHD | MEDICAL DIRECTOR

While medical teams at life science agencies are table stakes, the roles and responsibilities of the groups can vary widely. Those that can offer broad healthcare experience with deep expertise in specialized spaces such as oncology are key to building breakthrough brands.
A world where accelerated approvals are now commonplace
As a declared focus area for the ten largest pharmaceutical companies, oncology is one of the most exciting fields to support (McKinsey). However, the oncology sector often presents unique challenges that require unique expertise. Despite notable therapeutic successes, high unmet needs still exist in disease states that continue to suffer from poor patient outcomes, including colorectal cancer, pancreatic cancer, EGFR mutation, negative NSCLC, and other indications where chemotherapy is still the standard of care.
As new market entrants speed toward commercialization with dizzyingly aggressive launch timelines, experience having navigated similar launches becomes more critical to success. High percentages of products with Breakthrough Designation and Accelerated Approval contribute to these compressed timelines and shortened launch windows. Between 2012 and 2017, 95% of the oncology products approved by the FDA were expedited under at least one program, as shown below.
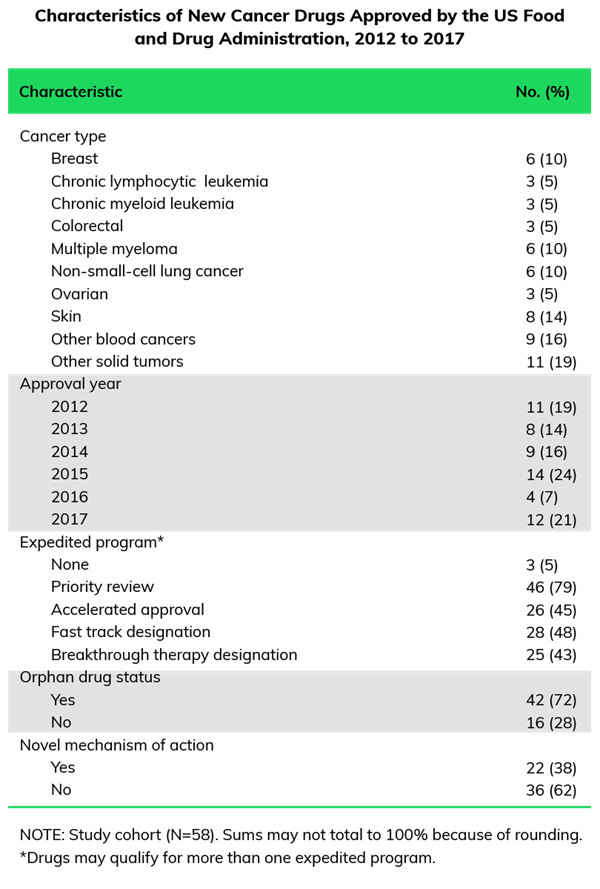
Under these conditions, an agile agency partner with an informed launch framework is beyond critical. The agency’s medical group makes essential contributions to these preparations by working with internal strategy and marketing partners to drill into data, clinical trials, and the overall competitive landscape to uncover clear clinical differentiators that inform the product positioning so that the launch brand is set up for long-term success.
Pat McGloin, Chief Client Officer at MERGE, adds that “Integrating the medical group early and often in launch preparations helps set up the brand for success, and in the frenetic pace of oncology innovation, there is no second chance at launch excellence. Further, we like to leverage our medical team as a strategic ally, providing the lexicon and sourcing ammunition to help clients navigate their internal MLR process while pushing for stronger claims that stay within the regulatory guardrails.”
In oncology, the medical team must be versatile and strategic
Asserting that an agency is an expert in “oncology” can sound impressive, but it can sometimes inadvertently minimize the true breadth and depth of knowledge and experience that is needed to provide credibility. Within the broad field of oncology, guidelines, treatment strategies, drug classes, and practices vary widely across tumor types and disease states. And beyond biology, we must remember to consider hematologists and oncologists as heterogeneous groups–with motivations, mindsets, and real-world practices that differ across specialties for both liquid and solid tumor treatment. Thus, the most effective medical groups retain talent that have a wide variety of experiences and expertise–spanning lymphomas, leukemias, lung, breast, GI, skin and prostate cancer, among other high-volume indications.
The pace of oncology innovation can lead to situations where clinical differentiation becomes more difficult to articulate. Crowded markets can be full of new pharmaceutical entrants which demonstrate incremental improvements in efficacy, safety, or both. In these instances, landing on a differentiated clinical positioning can require a thin-slicing of the data, a deep understanding of scientific nuance, and the ability to communicate these differences simply without being simplistic. So, the seasoned medical team will not only span the wide range of indications in oncology, but will also possess the depth of knowledge to ensure subject matter expertise in both the tumors being treated, and the technologies in development to treat them.
Therapeutic categories dominating recent growth and innovation in the oncology market include a number of novel cell and gene therapies. Chimeric antigen receptor (CAR) T-cell therapy, RNA therapy, viral vectors, and stem cell therapies represent market entrants that involve breakthrough therapies from complex manufacturing work streams to nuanced mechanisms of action (MOAs).

Precision medicine, driven by identification of oncology biomarkers and the use of companion diagnostics, and additional immuno-oncology advancements also contribute significantly to the complexity of innovation within the field of oncology.
Drugs with highly technical MOAs require the agency medical team to provide deep scientific understanding that informs both consumable education efforts and clinical differentiation. The rapid nature of oncology innovation provides a clear opportunity for marketing agencies to create needed educational content for hematologists, oncologists and other HCPs who may not be able to stay abreast of all of the new technology. And this education can, and often should, begin with unbranded efforts, with MOAs and compound information communicated in pre-commercial, pre-clinical settings, to prime the market for uptake at launch.

Clinical differentiation is about superior efficacy — unless it’s not.
Case-in-point: My team and I worked with a client who was bringing a product to market for a rare, and sometimes deadly, condition that occurs tangentially to hematologic diseases. Their data was incredible, but the perception among hematologists/oncologists was that the condition was quite rare and generally not life-threatening; thus, treatment typically consisted of supportive care. Our task, on an accelerated timeline, was to develop a disease awareness campaign that would overcome this treatment inertia. The medical team worked closely with creative to create bold imagery combined with comprehensive, scientifically based messaging around a biological pathway that HCPs were familiar with, but likely haven’t thought about in detail since medical school. The unbranded campaign served to heighten awareness of the deadly consequence of this condition, as well as to prime hematologists to understand the nuances of the pathway upon which drugs could intervene.
The ultimate goal is to deliver the best outcomes possible for patients and their caregivers, and we understand exactly what's involved in getting an oncology product across the finish line. Incorporating the medical team’s input early and often not only gives access to the full brand picture, but it also enables maximum efficiency to ensure a smooth submission and launch process. By partnering with the medical team one is able to push the very limits of creativity, while keeping firmly grounded in the science at hand and ultimately making your brand that much stronger.
At MERGE, we help life science brands engage with patients and HCPs during the moments that matter most. Contact us today to find out more.